The importance of pH: The key to balanced chemistry
pH, short for “potentia Hydrogenii,” is a term we often hear in the context of chemistry and biology. Although it may seem like a simple concept, pH plays a vital role in countless processes in our world. In this article, we take a closer look at the importance of pH, how it is measured and what role it plays in our daily lives.
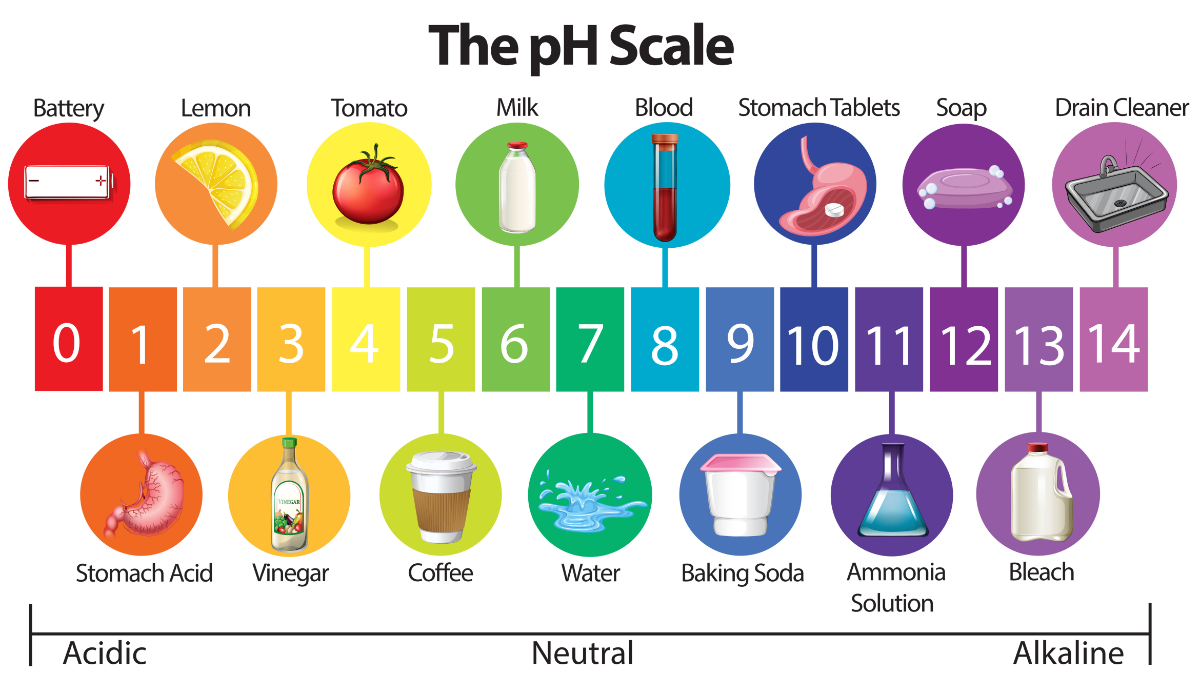
What is pH?
pH is a measure of the acidity or alkalinity of a solution. It is based on the concentration of hydrogen ions (H+) in that solution. The pH scale runs from 0 to 14, with 7 being the neutral point. Values less than 7 are considered acidic, while values greater than 7 are alkaline or basic.
The meaning of pH in nature:
pH plays an essential role in the natural world. It affects the growth and survival of plants, animals and microorganisms. For example, in agriculture, soil pH is of great importance. Different plants thrive at specific pH levels, and the right pH can affect the availability of nutrients in the soil.
pH in our daily life:
pH is not only important in nature, but also in our daily lives. Here are some examples:
1. Water Quality: The pH of water affects its taste, safety and usability. For example, water with a low pH can be corrosive and corrode metal pipes, while water with a high pH can cause mineral deposits.
2. Food preparation: The pH is an important factor in cooking and storing food. It affects the taste, texture and preservation of food. For example, preserving food requires an acidic environment to prevent the growth of bacteria.
3. Cosmetics and personal care: pH is a crucial consideration when formulating cosmetic products such as soaps, shampoos and creams. The correct pH helps the products to be effective and not to irritate the skin.
4. Swimming Pools: pH regulation is essential for maintaining water quality in swimming pools. An incorrect pH can promote bacterial growth, reduce the effectiveness of sanitizers, and cause eye and skin irritation.
pH measurement and regulation:
pH can be measured using pH indicators, pH test strips or electronic pH meters. These instruments provide a numerical value that indicates whether a solution is acidic, neutral, or basic. To keep the pH in a desired range, pH-regulating substances can be added. For example, adding an acid can lower the pH, while adding a base raises the pH.
Conclusion:
pH is a fundamental concept in chemistry and plays an important role in various aspects of our lives. Understanding pH allows us to better understand nature, ensure water quality, prepare food safely, formulate effective cosmetic products and much more. So, let’s not overlook pH, as it is the key to balanced chemistry.
Deel dit artikel:
Op zoek naar kwalitatief laboratoriummateriaal?
Om een overzicht van al onze producten te vinden kan u ook onze brochure consulteren.
